RESEARCH NEWS
Findings from several of our studies of pregnancy and lactation associated osteoporosis (PLO) were presented at the American Society for Bone and Mineral Research (ASBMR) 2024 Annual Meeting – including studies of genetic etiologies, biopsy-based bone remodeling, and treatment protocols for PLO. Congratulations to Dany El-Najjar (Columbia MSY4) and Lauren Lynch, MD, PhD who were both honored with ASBMR Young Investigator Awards for presentations of this work at the 2024 Annual Meeting!
NOW RECRUITING!
ABOUT OUR RESEARCHObservational Studies
Pregnancy and Lactation Associated Osteoporosis Studies
Pregnancy and Lactation Associated Osteoporosis (PLO) is a rare condition in which young women sustain low trauma fractures during or soon after pregnancy, or during breastfeeding. Because PLO is rare, we know relatively little about its clinical features, causes, and prognosis. As medical researchers at CUIMC, we are very interested in studying the causes of and potential treatments for PLO. If you are eligible, we encourage you to consider participating in one or more different aspects of our research.
Bone Density Follow Up Study: Clinical Observational Study of Pregnancy and Lactation Associated Osteoporosis
Patients who choose to have clinical follow-up and/or medication treatment through the Metabolic Bone Diseases Program at CUIMC can contact the PATIENT CARE section of the EOC at CUIMC. For patients who elect this clinical care option, this study includes bone density testing every six months—in addition to usual clinical care—as well as specialized laboratory studies of bone metabolism.
FUNDED STUDIESThe Center’s tools of inquiry include advanced three-dimensional (3D) high resolution bone-imaging equipment and analysis techniques, evaluation of bone biopsy samples, laboratory investigation of bone cells, and analysis of patients’ DNA to learn about potential genetic etiologies of Early-Onset Osteoporosis

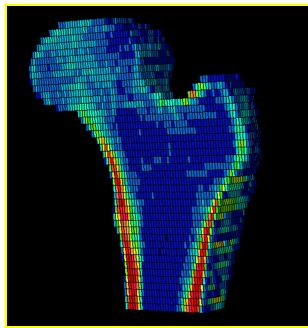
1. High resolution peripheral computed tomography (HR-pQCT):
This imaging technique provides a non-invasive method to visualize the microarchitecture of the radius and tibia bones.
Image analyses are conducted in collaboration with the laboratory of X. Edward Guo, PhD, Stanley Dicker Professor of Biomedical Engineering and Chair, Department of Biomedical Engineering, Columbia University.
2. Central quantitative computed tomography:
This imaging technique provides a non-invasive method to study the 3-dimensional structure of the spine and hip.
Image analyses are conducted in collaboration with Thomas Lang, PhD, Professor of Radiology and Biomedical Imaging, School of Medicine, UCSF.
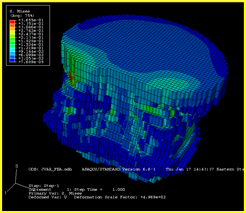
Structural analyses (finite element analyses) of the central QCT images of the spine and hip
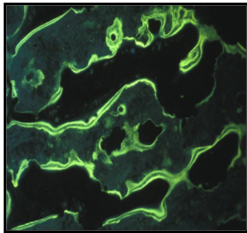
3. Bone biopsy studies:
Bone biopsy samples allow us to examine bone structure and remodeling as well as bone cell activity. Studies are conducted in collaboration with: David Dempster, PhD, Professor of Clinical Pathology at CUIMC, Stavroula Kousteni, PhD, Professor of Physiology & Cellular Biophysics at CUIMC, Matthew Allen, PhD, Professor Anatomy, Cell Biology & Physiology, Indiana University, and Ralph Muller, PhD, Professor of Biomechanics and Head of the Laboratory for Bone Biomechanics, ETH Zurich.
Within the bone biopsy samples, bone surfaces can be visualized and bone formation rate can be measured.
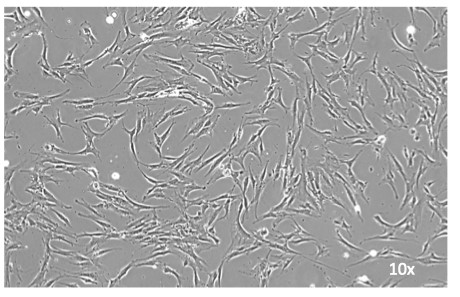
Bone cells obtained from the bone biopsy samples, grown in culture in the laboratory of Stavroula Kousteni, PhD, CUIMC.
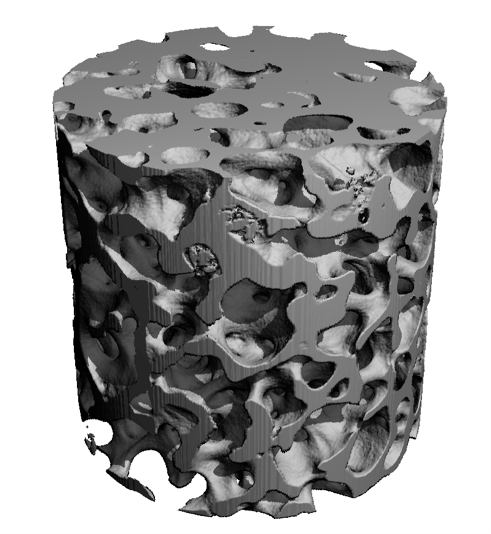
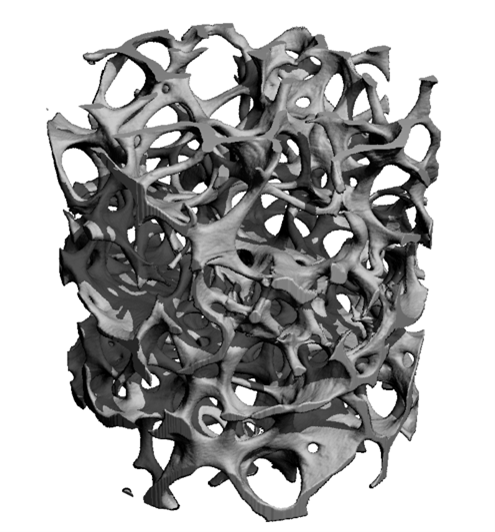
MicroCT analyses of bone biopsy samples, performed in the laboratory of Ralph Muller, PhD, ETH Zurich.
Healthy Bone
Osteoporotic Bone
4. Hormone and biochemistry studies:
In the Biomarkers Core Laboratory (BCL) at the Irving Institute for Clinical and Translational Research, we assess hormonal and biochemical characteristics of osteoporosis and biomarkers of medication response.5. Genetic Studies:
We work in collaboration with Columbia’s Center for Precision Medicine and Genomics to learn more about genetic causes of bone fragility in patients with early-onset osteoporosis.
Our research program has received funding from the US Food and Drug Administration (FDA), the National Institutes of Health (NIH), industry sponsors, the Simon-Strauss Foundation and the Thomas L. Kempner, Jr. and Katheryn C. Patterson Foundation.
Click here for More..
The following investigations are ongoing at the EOC.
Principal Investigators: Drs. Elizabeth Shane and Adi Cohen
- Romosozumab for Premenopausal Idiopathic Osteoporosis
The goal of this study is to characterize the effects of 24 months of treatment with romosozumab followed by denosumab on bone mass and remodeling in premenopausal women with idiopathic osteoporosis. This study has been approved by the Columbia University Institutional Review Board (IRB#AAAT1202). This study is closed to enrollment.
- Pregnancy and Lactation Associated Osteoporosis: Bone Microstructure and Metabolism, Genotypic Characteristics, Natural History and Biomarkers of Disease Severity
This study, funded by the FDA Office of Orphan Products Development through the Natural History Grants Program for rare diseases in 2017, aims to define the skeletal structure, bone metabolism, hormonal and genetic characteristics of women with PLO as an essential step toward the design of protocols to test targeted approaches to improve skeletal recovery and bone quality. This study has been approved by the Columbia University Institutional Review Board (IRB#AAAR0960).
- Personalized Investigation of Disease Mechanisms and Prediction of Medication Responsiveness in Women with Pregnancy and Lactation Associated Osteoporosis.
This study, funded by a Precision Medicine Pilot Award from Columbia’s Irving Institute for Clinical and Translational Research in 2018, allows us to perform individual functional and genetic analyses of bone forming cells (primary osteoblasts) obtained from PLO patients’ bone biopsy samples. This study has been approved by the Columbia University Institutional Review Board (IRB#AAAR0960).
- Bone Density Follow Up Study: Clinical Observational Study of Pregnancy and Lactation Associated Osteoporosis
For those patients who choose to have clinical follow up and/or medication treatment through the EOC at CUIMC, this study includes bone density testing every six months—in addition to usual clinical care—as well as specialized laboratory studies of bone metabolism. This study has been approved by the Columbia University Institutional Review Board (IRB#AAAR9157).
ONGOING RESEARCH WITH COMPLETED ENROLLMENT
PUBLICATIONSClick here for More..
- Teriparatide for Idiopathic Osteoporosis in Premenopausal Women: A Phase 2 Study
This study, funded by the FDA in 2010, is a randomized controlled trial of teriparatide for idiopathic osteoporosis. This study is closed to enrollment.
- Phase 2B Study of Denosumab to Prevent Bone Loss in Idiopathic Osteoporosis in Premenopausal Women Treated with Teriparatide
This study, funded by the FDA in 2016, is an extension to the Phase 2 teriparatide study above, and is closed to enrollment. The goal of the study is to characterize the effects of denosumab on bone mass and remodeling in premenopausal women with idiopathic osteoporosis who have completed teriparatide.
- Bisphosphonates for Prevention of Post-Denosumab Bone Loss in Premenopausal Women with Idiopathic Osteoporosis
This study, funded by Amgen, aims to determine whether transitioning to bisphosphonates prevents increases in bone remodeling and decreases in bone density in premenopausal women with idiopathic osteoporosis who have completed a course of teriparatide followed by one to three years of denosumab.
- IGF-1, bone turnover and response to teriparatide in premenopausal women with idiopathic osteoporosis.
This project, funded by the NIH in 2013, investigates the contribution of insulin like growth factor 1 (IGF-1) resistance to the pathogenesis of premenopausal idiopathic osteoporosis and the response to teriparatide.
Click here for More..
- Kamanda-Kosseh M, Shiau S, Agarwal S, Kondapalli A, Colon I, Kil N, Bucovsky M, Lappe JM, Stubby J, Shane E, Cohen A. Bisphosphonates maintain BMD after sequential teriparatide and denosumab in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab 2025 Feb; 110(3):e791-e801.
- Agarwal S, El-Najjar D, Kondapalli A, Kil N, Kamada-Kosseh M, Bucovsky M, Colon I, Lappe JM, Stubby J, Recker RR, Guo XE, Shane E, Cohen A. HR-pQCT Reveals Marked Trabecular and Cortical Structural Deficits in Women with Pregnancy and Lactation associated Osteoporosis (PLO), J Bone Miner Res 2024 Dec; 40(1): 38-49.
- Kondapalli A, Kamanda-Kosseh M, Williams JM, Shiau S, Bucovsky M, Colon I, Shane E, Cohen A. Clinical characteristics of pregnancy and lactation associated osteoporosis: An online survey study. Osteoporosis International 2023 Aug; 34(8):1477-89.
- Agarwal S, Shiau S, Kamanda-Kosseh M, Bucovsky M, Kil N, Lappe JM, Stubby J, Recker RR, Guo XE, Shane E, Cohen A. Teriparatide followed by denosumab in premenopausal idiopathic osteoporosis: Bone microstructure and strength by HR-pQCT. J Bone Miner Res. 2023 Jan; 38(1): 35-47.
- Agarwal S, Shane E, Lang T, Shiau S, Kamanda-Kosseh M, Bucovsky M, Lappe JM, Stubby J, Recker RR, Hu Y, Wang Z, Guo XE, Cohen A. Spine volumetric BMD and strength in premenopausal idiopathic osteoporosis: Effect of teriparatide followed by denosumab. J Clin Endocrinol Metab 2022 Jun; 107(7): e2690-e2701.
- Shane E, Shiau S, Recker RR, Lappe JM, Agarwal S, Kamanda-Kosseh M, Bucovsky M, Stubby J, Cohen A. Denosumab after teriparatide in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab 2022 Mar; 107(4): e1528-e1540.
- Goetz TG, Nair N, Shiau S, Recker RR, Lappe JM, Dempster DW, Zhou H, Zhao B, Guo X, Shen W, Nickolas TL, Kamanda-Kosseh M, Bucovsky M, Stubby J, Shane E, Cohen A. In premenopausal women with idiopathic osteoporosis, lower bone formation is associated with higher body fat and higher IGF-1. Osteoporosis International, 2022 Mar; 33(3): 659-672.
- Cohen A, Hostyk J, Baugh EH, Buchovecky CM, Aggarwal VS, Recker RR, Lappe JM, Dempster DW, Zhou H, Kamanda-Kosseh M, Bucovsky M, Stubby J, Goldstein DB, Shane E. Whole exome sequencing reveals potentially pathogenic variants in a small subset of premenopausal women with idiopathic osteoporosis. Bone 2022 Jan 154:116253.
- Cohen A, Shiau S, Nair N, Recker RR, Lappe JM, Dempster DW, Nickolas TL, Zhou H, Agarwal S, Kamanda-Kosseh M, Bucovsky M, Williams JM, McMahon DJ, Stubby J, Shane E. Effect of Teriparatide on Bone Remodeling and Density in Premenopausal Idiopathic Osteoporosis: A Phase II Trial. J Clin Endocrinol Metab 2020 Oct; 105(10).
- Cohen A, Kamanda-Kosseh M, Dempster DW, Zhou H, Muller R, Goff E, Colon I, Bucovsky M, Stubby J, Nickolas TL, Stein EM, Recker RR, Lappe JM, Shane E. Women with pregnancy and lactation associated osteoporosis (PLO) have low bone remodeling rates at the tissue level. J Bone Miner Res. 2019 Sep;34(9): 1552-1561.
- Cohen A, Kousteni S, Bisikirska B, Shah JG, Manavalan JS, Recker RR, Lappe J, Dempster DW, Zhou H, McMahon DJ, Bucovsky M, Kamanda-Kosseh M, Stubby J, Shane E. IGF-1 Receptor expression on circulating osteoblast progenitor cells predicts tissue-based bone formation rate and response to teriparatide in premenopausal women with idiopathic osteoporosis. J Bone Miner Res. 2017 June; 32(6):1267-1273.
- Cohen A, Kamanda-Kosseh M, Recker RR, Lappe JM, Dempster DW, Zhou H, Cremers S, Bucovsky M, Stubby J, Shane E. Bone density after teriparatide discontinuation in premenopausal idiopathic osteoporosis. J Clin Endocrinol Metab. 2015 Nov; 100(11):4208-14.
- Nishiyama KK, Cohen A, Young P, Wang J, Lappe JM, Guo XE, Dempster DW, Recker RR, Shane E. Teriparatide increases strength of the peripheral skeleton in premenopausal women with idiopathic osteoporosis: a pilot HR-pQCT study. J Clin Endocrinol Metab 2014; 99(7):2418-25.
- Cohen A, Dempster DW, Recker RR, Lappe JM, Zhou H, Zwahlen A, Muller R, Zhao B, Guo, X, Lang T, Saeed I, Liu XS, Gou XE, Cremers S, Rosen CJ, Stein EM, Nickolas TL, McMahon DJ, Young P, Shane E. Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: a transiliac bone biopsy study. J Clin Endocrinol Metab. 2013; 98(6):2562-72.
- Cohen A, Stein EM, Recker RR, Lappe JM, Dempster DW, Zhou H, Cremers S, McMahon DJ, Nickolas TL, Muller R, Zwahlen A, Young P, Stubby J, Shane E, Teriparatide for idiopathic osteoporosis in premenopausal women: a pilot study. J Clin Endocrinol Metab. 2013; 98(5):1971-81.
- Cohen A, Lang TF, McMahon DJ, Liu XS, Guo XE, Zhang C, Stein EM, Dempster DW, Young P, Saeed I, Lappe JM, Recker RR, Shane E. Central QCT reveals lower volumetric BMD and stiffness in premenopausal women with idiopathic osteoporosis, regardless of fracture history.J Clin Endocrinol Metab 2012;97(11):4244-52.
- Cohen A, Dempster DW, Stein EM, Nickolas TL, Zhou H, McMahon DJ, Muller R, Kohler T, Zwahlen A, Lappe JM, Young P, Recker RR, Shane E. Increased marrow adiposity in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab 2012; 97(8): 2782-91.
- Cohen A, Recker RR, Lappe J, Dempster DW, Cremers S, McMahon DJ, Stein EM, Fleischer J, Rosen CJ, Rogers H, Staron RB, LeMaster J, Shane E. Premenopausal women with idiopathic low trauma fractures and/or low bone mineral density. Osteoporosis International. 2012; 23(1): 171-82.
- Cohen A, Dempster D, Recker R, Stein EM, J L, Zhou H, Wirth AJ, van Lenthe GH, Kohler T, Zwahlen A, Muller R, Rosen CJ, Cremers S, Nickolas TL, DJ M, Rogers H, Staron RB, Lemaster J, Shane E. Abnormal bone microarchitecture and evidence of osteoblast dysfunction in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2011; 96(10): 3095-105.
- Liu XS, Cohen A, Shane E, Stein E, Rogers H, Kokolus SL, Yin PT, McMahon DJ, Lappe JM, Recker RR, Guo XE. Individual trabeculae segmentation (ITS)-based morphological analyses of high resolution peripheral quantitative computed tomography images detect abnormal trabecular plate and rod microarchitecture in premenopausal women with idiopathic osteoporosis. J Bone Mineral Res 2010; 25(7): 1496-1505.
- Cohen A, Liu XS, McMahon DJ, Rogers HF, LeMaster J, Recker RR, Lappe JM, Guo XE, Shane E. Bone microarchitecture and stiffness in premenopausal women with osteoporosis. J Clin Endocrinol Metab 2009; 94(11):4351-60.
- Cohen A, Fleischer J, Freeby MJ, McMahon DJ, Irani D, Shane E. Clinical characteristics and medication use among premenopausal women with osteoporosis and low BMD: the experience of an osteoporosis referral center. Journal of Women’s Health 2009; 18(1):79-84.